Mapping Enantiospecific Reaction Kinetics on Surface Structure Spread Single Crystals (S4C)
Aaron D. Reinicker, Matthew Payne, Burcu Karagoz
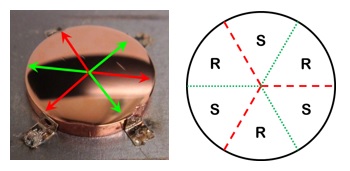
Many chemical reactions occurring on surfaces are structure sensitive in the sense that their rates depend of the atomic structure or crystallographic orientation of the surfaces on which they occur. The root cause of structure sensitivity is the dependence of reaction rate constants on the Miller indices, (hkl), of the crystal plane, kihkl. Comparisons of reaction rates on flat single crystal surfaces were first used to demonstrate the structure sensitivity of various reactions, however, a crystal surface with a single crystallographic orientation allows study of only one surface structure at a time. Comprehensive study of structure sensitivity across the entire stereographic projection of surface orientations is intractable using this approach. Surface Structure Spread Single Crystals (S4Cs) (Figure 1) that are curved expose a distribution of surface orientations that spans an entire region of the stereographic triangle [1]. These allow surfaces with a continuous distribution of different orientations to be studied in one experiment under identical conditions. In order to span all possible surface orientations, a set of six Cu S4Cs has been created that spans the entire stereographic triangle.
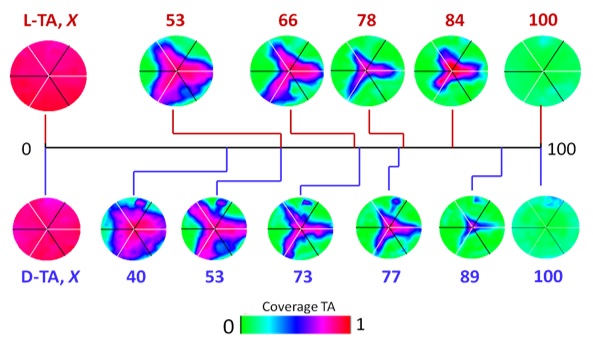
The kinetics of tartaric acid (TA) and aspartic acid (Asp) thermal decomposition on Cu(hkl) surface are known to be structure sensitive and, because both molecules are chiral, their decomposition kinetics depend on the chirality of the Cu surfaces. Quenched isothermal TPRS experiments on Cu(111) and Cu(100) S4Cs allow the decomposition of D- and L-TA to be interrupted at various extents of reaction, meaning that decomposition is complete on some surface orientations but has not even begun on others. By mapping the O 1s XPS signal across the S4C we can determine the local TA coverage remaining at all possible surface orientations spanned by the S4C (Figure 2). Studies of L- and D- TA decomposition on Cu(111) S4C show that decomposition occurs most rapidly on (100) step edges and least rapidly on the (110) steps and (111) terrace. However, the kinetics are not detectably sensitive to the chirality of the surface. More recent work using a Cu(100) S4C has revealed the enantiospecificity of D- and L-TA decomposition and provided an unprecedentedly detail understanding of its structure sensitivity. Ongoing work is studying TA and Asp decomposition kinetics across all six of the Cu(hkl) S4Cs and will be used to map the rate constants ki(hkl-R) and ki(hkl-S) across the entire stereographic projection. These can then be used to identify the surface structures that are most enantioselective.