Alloy Catalysis Across Composition Space - CuxAuyPd1-x-y
Irem Sen, Peter Kondratyuk
Study of catalysis by multicomponent materials is hampered by the need to perform catalyst preparation, characterization and reactivity measurements for large numbers of discrete catalyst compositions. Composition Spread Alloy Films (CSAFs) enable such studies to span composition space comprehensively. CSAFs are thin multicomponent films that have composition gradients parallel to their surfaces, AxByC1-x-y with x = 0 → 1 and y = 0 → 1-x. Many otherwise intractable fundamental scientific problems in alloy science and catalysis can be effectively addressed through use of CSAFs as high throughput materials libraries.
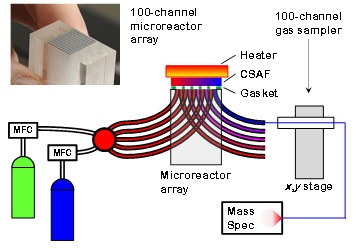
Study of alloy catalysis across composition space using a CSAF requires a multichannel reactor system that can be used to run steady-state catalytic reactions at many different alloy compositions on the CSAF. We have developed a 100-channel microreactor array reactor that can sample product distributions from 100 different alloy catalysts in the space of about 10 minutes and for temperatures in the range 300 - 600 K.[1] Figure 1 is an illustration of the 100-channel microreactor array system. At left the gas-handling system mixes and delivers gasses through 100 (5 illustrated) capillaries tubes. Within the glass microreactor block the gases are channeled to 100 isolated regions (5 illustrated) of the CSAF each of which isolates a different alloy catalyst composition. The products from each composition on the CSAF are extracted through 100 capillary channels (5 illustrated) and sampled sequentially by a smaller sampling capillary that transports the gases to a mass spectrometer.
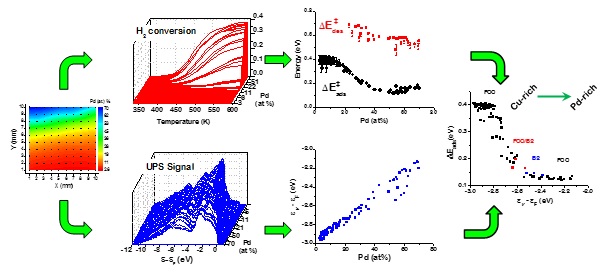
The kinetics of H2-D2 exchange catalysis have been measured at 100 different compositions across a binary CuxPd1-x CSAF and 100 compositions across a ternary CuxAuyPd1-x-y CSAF at temperatures in the range 300 - 600 K (Figure 2).[2,3] Microkinetic analysis of the data has allowed estimation of the activation barriers for the dissociative adsorption, ΔE_ads^? (x,y), and the recombinative desorption, ΔE‡des(x,y), of H2 both as functions of alloy composition. In parallel with these measurements of catalytic activity, UV and x-ray photoemission spectroscopy have been used to map the average electronic valence band energies across alloy composition space, Ēev(x,y), (Figure 2). These datasets are the basis for experimentally derived correlations between the reaction barriers and alloy electronic structure, ΔE‡ads (Ēev). Having developed these tools for high-throughput alloy catalysis, we are launching studies of processes such as ethylene and acetylene hydrogenation, hydrogen peroxide synthesis, and oxygen reduction reactions across various alloy composition spaces.
Related Publications
- P. Kondratyuk, G. Gumuslu, S. Shukla, J.B. Miller, B.D. Morreale, A.J. Gellman "A Microreactor Array for Spatially-Resolved Measurement of Catalytic Activity on Composition Spread Alloy Films: a Device for High-Throughput Catalyst Evaluation" Journal of Catalysis; 2013, 300, 55-62. View paper
- J.R. Boes, G. Gumuslu, J.B. Miller, A.J. Gellman, J.R. Kitchin, "Estimating bulk composition dependent H2 dissociative adsorption energies on CuxPd1-x alloy (111) surfaces" ACS Catalysis; 2015, 5(2), 1020-1026 . View paper
- G. Gumuslu, P. Kondratyuk, J.R. Boes, B. Morreale, J.B. Miller, J.R. Kitchin, A.J. Gellman, "Correlation of electronic structure and H2-D2 exchange activity across CuxPd1-x composition space" ACS Catalysis; 2015, 5, 3137-3147 . View paper